Research
Latest Research
Our latest clinical trial of StarStarter Rx (aka ABM-01) showed robust reductions in social anxiety. For more detail, read our research poster through the below link.



Published Research
Our game-based approach to the cognitive training technique Attention Bias Modification (ABM) has been widely published in leading peer-reviewed research journals.
Jennifer de Rutte, Sarah Myruski, Elizabeth Davis, Abigail Findley, Tracy A. Dennis-Tiwary
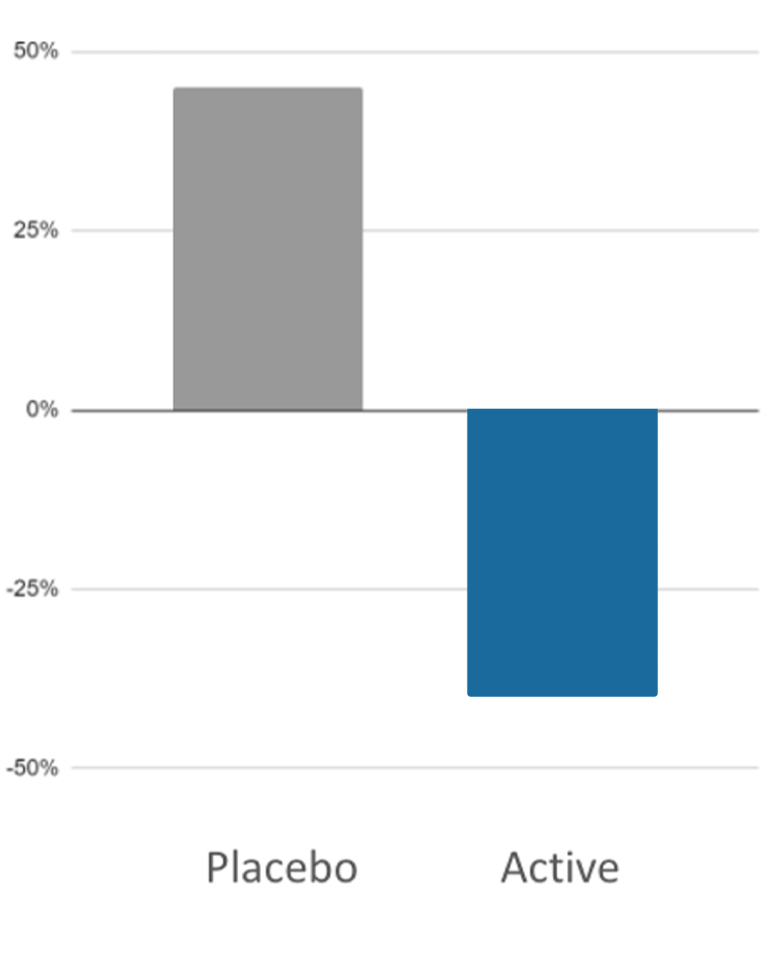
Purpose: A longitudinal clinical trial pilot study to investigate gamified attention bias modification (ABM) as an efficacious digital therapeutic tool for the reduction of social anxiety disorder symptom severity
Study Population: 104 patients aged 22-64 who reported elevated SAD symptoms (scores of 7 and above) on the 3-item Mini-Social Phobia Inventory – Revised
Administration: Four weekly 12-minute sessions across 4 weeks
Primary Endpoints:
- Self-reported Social Anxiety for adults via The Liebowitz SAD Scale Self-Report (LSAS-SR)
Results:



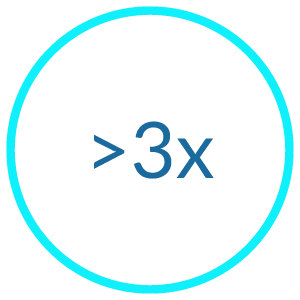
Dennis-Tiwary, T. A., Denefrio, S., and Gelber, S. (2017)
Purpose: Proof-of-concept longitudinal study to investigate potential for game-based therapeutic targeting pregnancy-related anxiety
Study Population: Patients aged 23-45 in their 19th-29th week of pregnancy
Administration: 30 minutes per week for 4 weeks with placebo arm
Primary Endpoints:
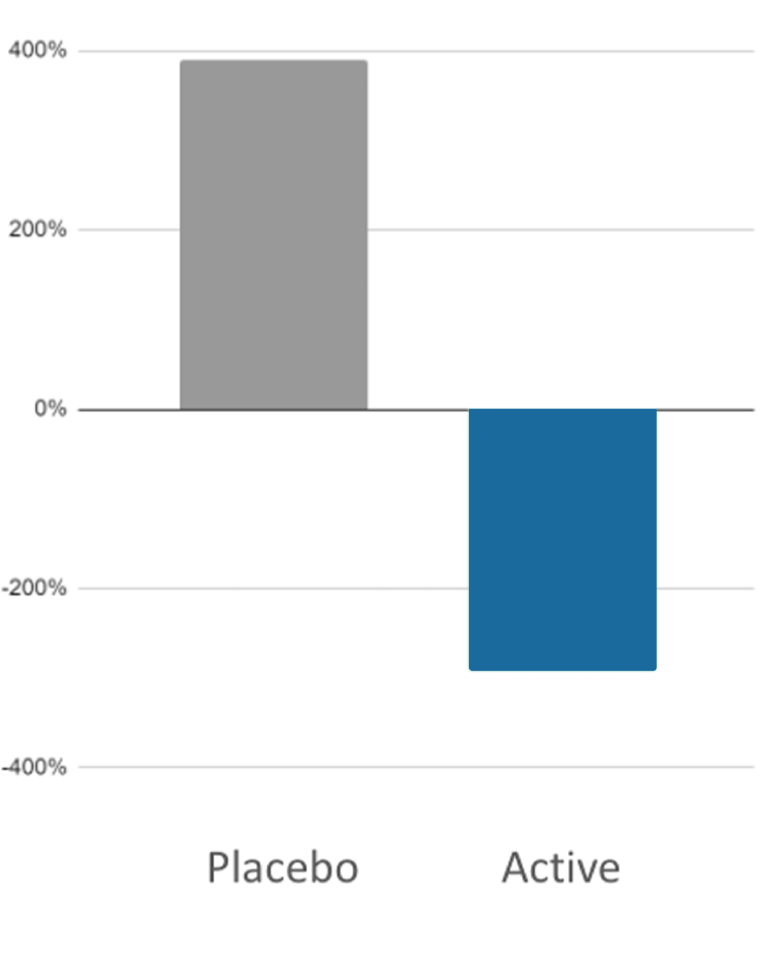
- Salivary cortisol following exposure to standardized stressor (AUC1)
- Cognitive signs of anxiety (Dot Probe Task Measure)
Results:
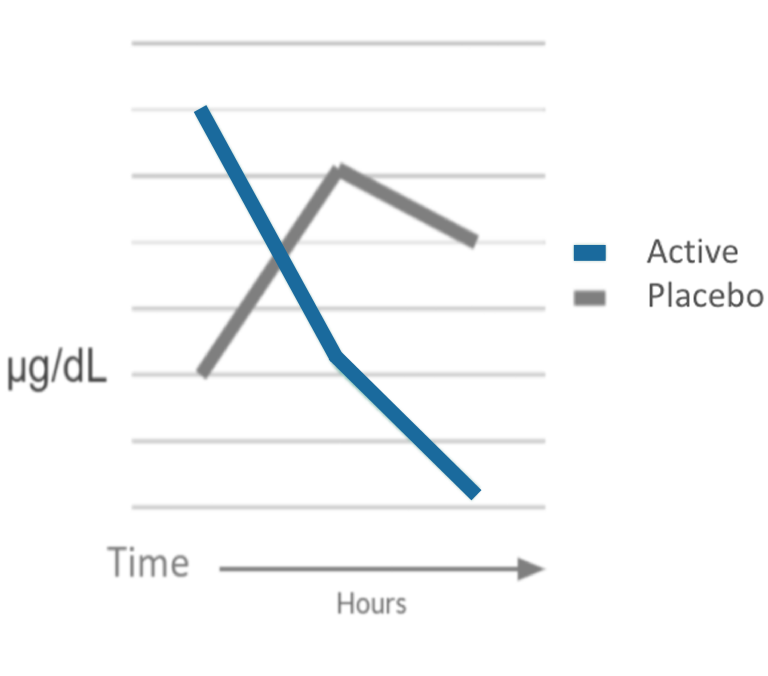

Charvet, L., George, A., Cho, H., Crupp, L.B. & Dennis-Tiwary, T. A. (2021)
Purpose: Proof-of-concept longitudinal study to investigate potential for game-based therapeutic targeting MS-related anxiety
Study Population: Patients (ages 12-24) with pediatric-onset Multiple Sclerosis
Administration: 30 minutes per week over 4 weeks
Primary Endpoints:
- Self-reported anxiety for adults (Beck Anxiety Inventory)
- Self-reported anxiety for youth (Multidimensional Anxiety Scale for Children)
- Self-reported mood (Positive and Negative Affect Schedule)
Results:


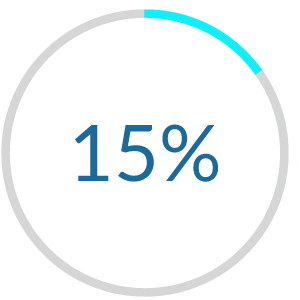
Dennis-Tiwary, T. A., and O’Toole, L., J. (2014)
Purpose: Two randomized controlled trials conducted to optimize gameplay dosage and serve as proof of concept studies for individuals with high trait anxiety
Study Population: Participants aged 18-38 with high trait anxiety, measured via State-Trait Anxiety Inventory (STAI)
Administration: Two separate single session studies at varying durations with placebo arms
Primary Endpoints:
- Self-reported state anxiety measured by State-Trait Anxiety Inventory (STAI)
- Cognitive signs of anxiety (Dot Probe Task Measure)
Results:
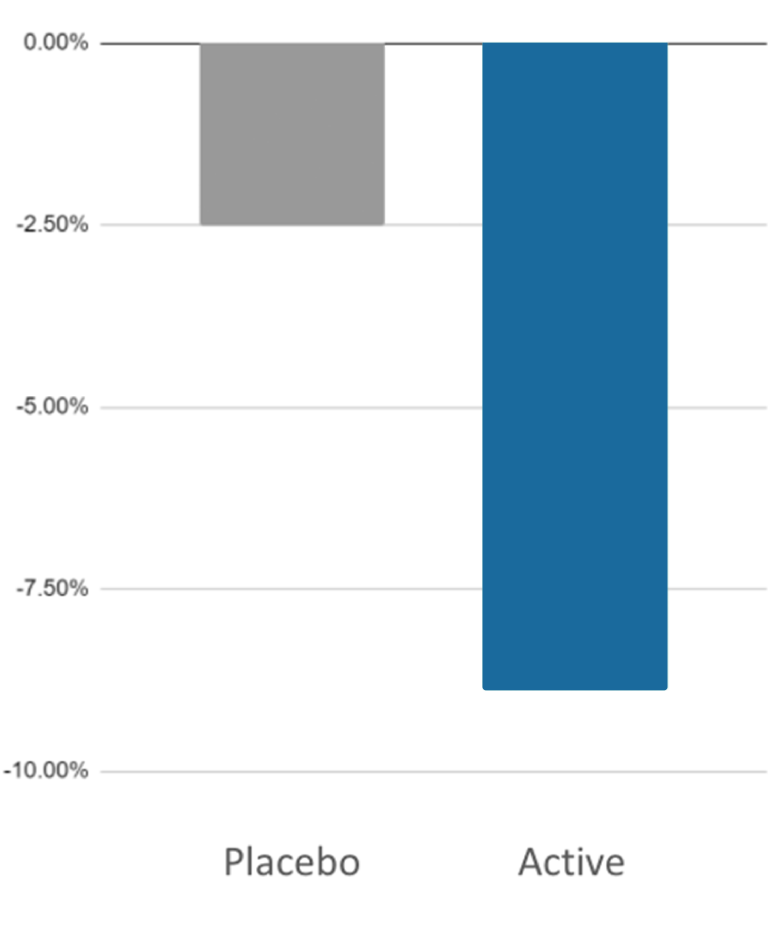
Short Study
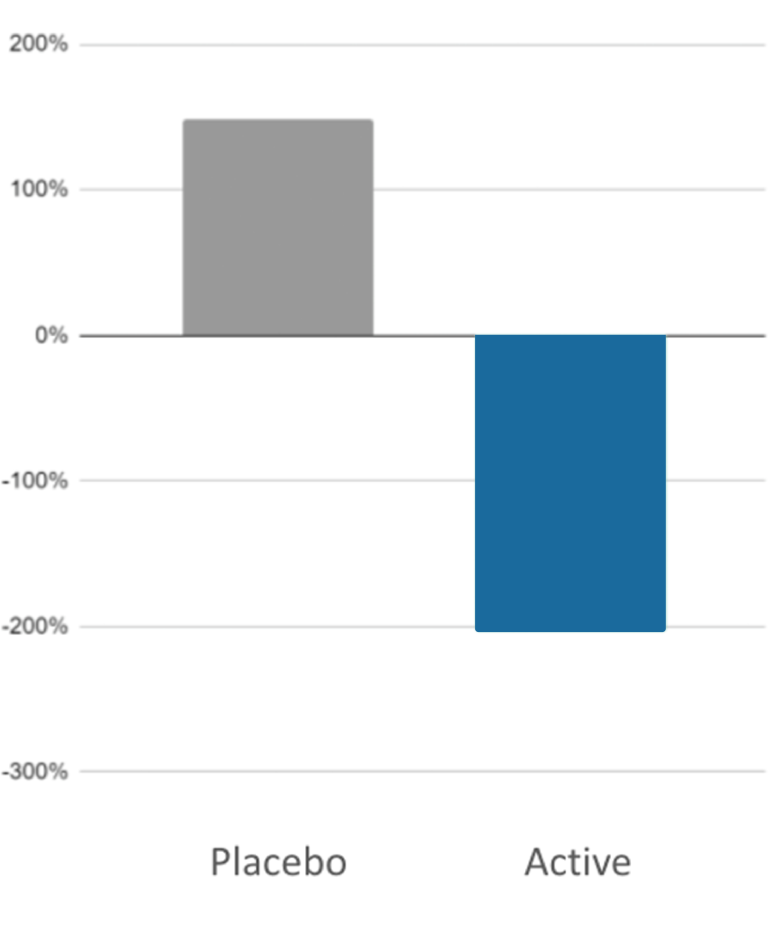
Long Study
Dennis-Tiwary, T. A.,Egan. L. J., Babkirk, S., and Denefrio, S. (2016)
Purpose: To map out individual cognitive differences among patient cohorts with high trait anxiety for game design optimization
Study Population: Participants aged 18-38 with high trait anxiety, measured via State-Trait Anxiety Inventory (STAI)
Administration: Single session study with placebo arm
Primary Endpoints:
- Cognitive control; event related potentials (ERPs) measured via Negative Deflection After Visual Stimulus (N2)
- Stress resilience measured via Trier Social Stress Test (TSST)
Results:
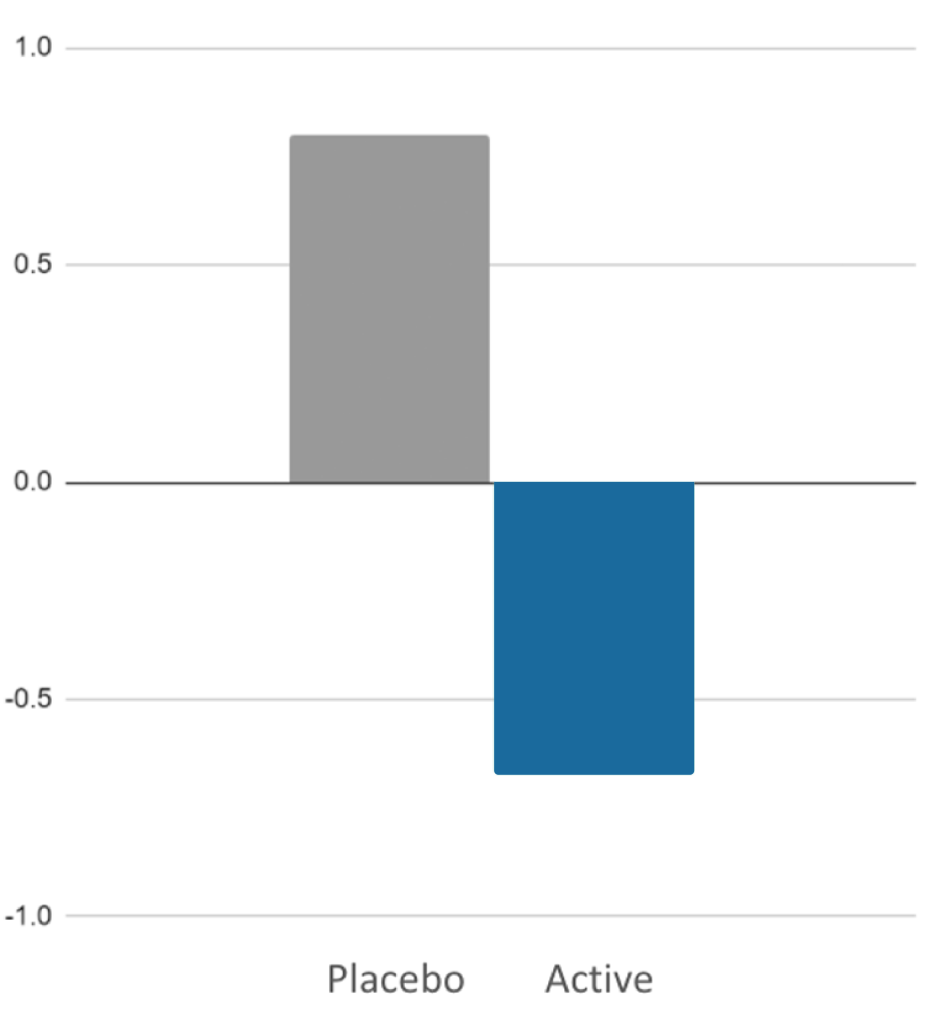
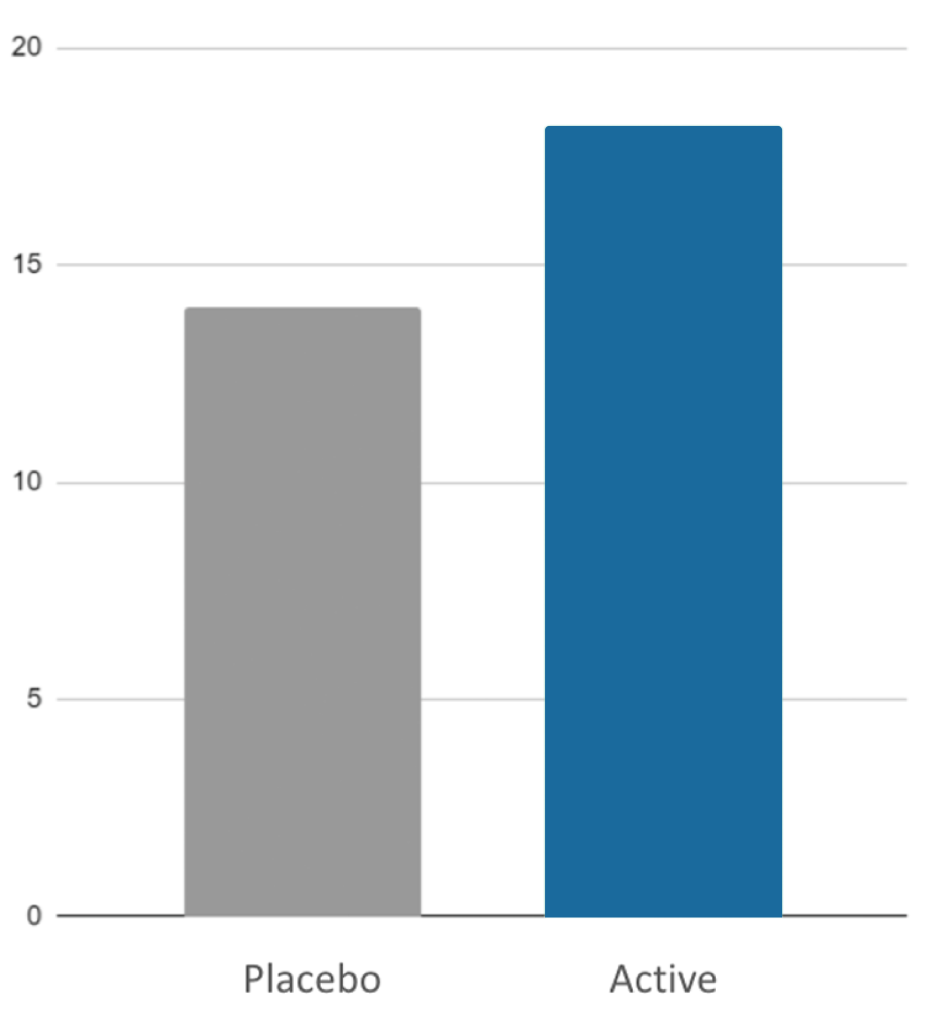
Myruski, S., Cho, H., Bikson, M., & Dennis-Tiwary, T. A. (2021)
Purpose: Proof-of-concept study to investigate potential for combination treatment of neurostimulation and game-based intervention for anxiety.
Study Population: Adults with normal to moderate levels of anxiety measured by DASS-21
Administration: Single session in combination with tDCS neurostimulation headset; placebo tDCS arm
Primary Endpoints:
- Self-reported anxiety (Analog Mood Scale)
- Cognitive signs of anxiety (Dot Probe Task Measure)
Results:


Luehring-Jone, P., Louis, C., Dennis-Tiwary, T. A., and Erblich, J. (2017)
Purpose: Proof-of-concept study to investigate potential for game-based intervention for alcohol use disorder (AUD)
Study Population: Adult alcohol drinkers with varied responses to Alcohol Use Disorders Identification Test and Obsessive-Compulsive Drinking Scale. Participants excluded if they drink less than three times per week.
Administration: Single session with placebo arm
Primary Endpoints:
- Cue-induced alcohol craving
- Cognitive measures of preferential processing of alcohol-related information (Alcohol Stroop Task)
Results: